How To Calculate E Cell From Half Reactions
Cell reaction half overall electrochemistry class iit jee Calculating ecell Calculation of half cell potential electrochemistry 2 class 12
HOW to Calculate the E*cell of the half cell reactions - YouTube
Solved write the cell reaction and electrode half-reactions Reaction electrode cl2 ag solved reactions calculate transcribed problem Solved use the following half-reactions to write three
Reactions use write half following three cell reaction calculate spontaneous oxidizing each rank agents solved reducing answer
Occur electrode write notationDetermine transcribed Solved complete the half-reactions that occur at eachSolved determine the half-cell reactions and the overall.
How to calculate the e*cell of the half cell reactionsCell potential half calculation electrochemistry chemistry class Ecell calculatingHalf cell reaction, overall reaction (electrochemistry part 10 for cbse.

Calculate `e^(@)` of the following half -cell reaction at 298 k: `ag(nh
.
.

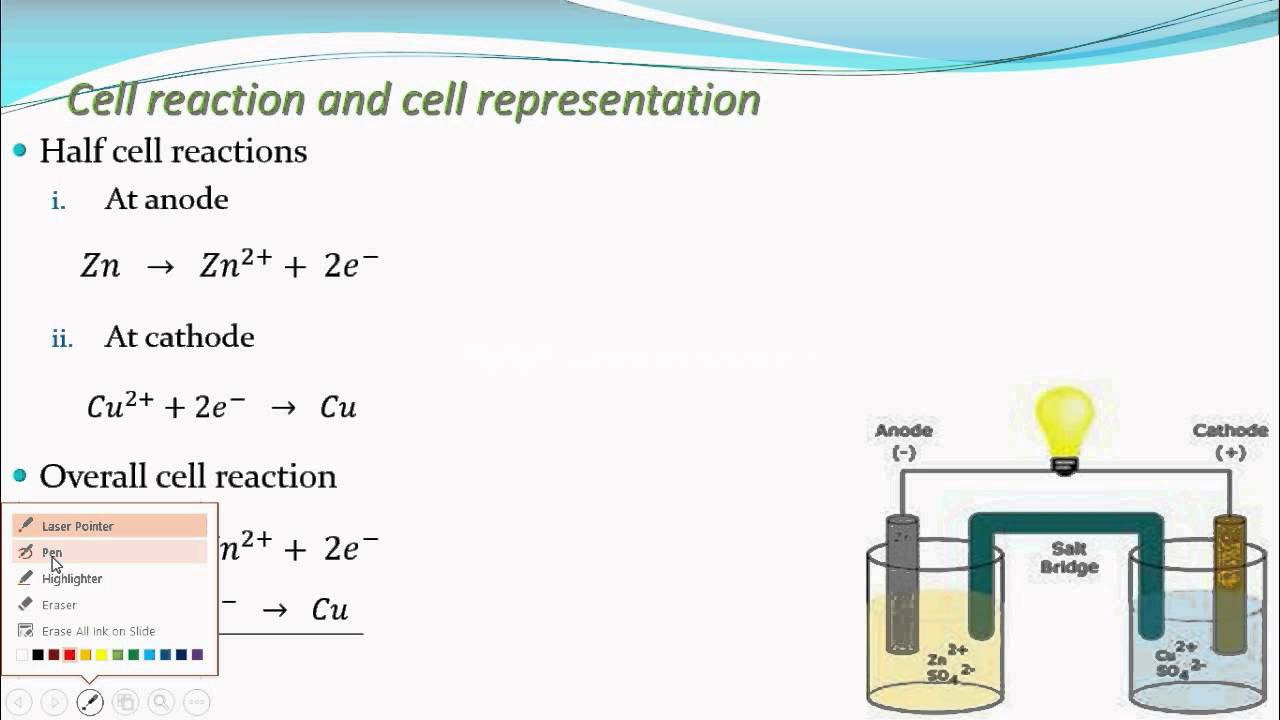
Half cell reaction, Overall reaction (Electrochemistry part 10 for CBSE
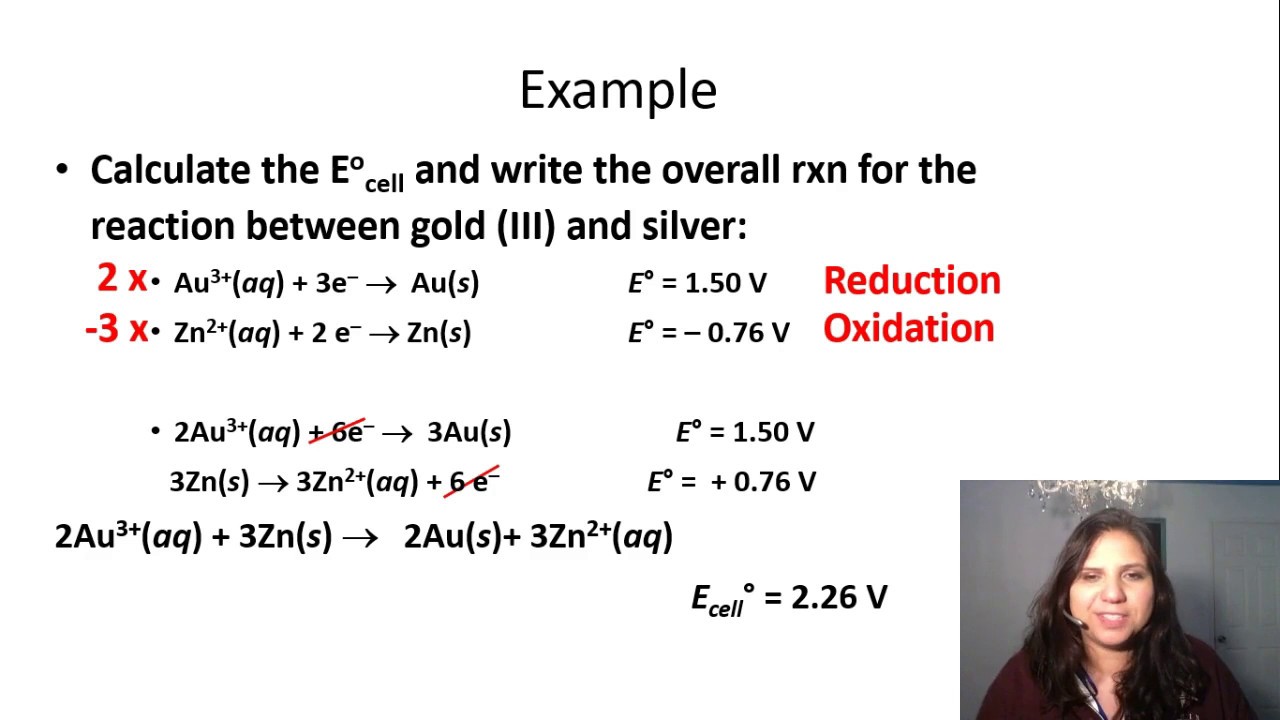
Calculating Ecell - YouTube

Solved Determine the half-cell reactions and the overall | Chegg.com

calculation of half cell potential electrochemistry 2 class 12

Solved Write the cell reaction and electrode half-reactions | Chegg.com

Solved Use the following half-reactions to write three | Chegg.com

calculate `E^(@)` of the following half -cell reaction at 298 K: `Ag(NH
